Background: Primary immune thrombocytopenia (ITP) is an acquired autoimmune bleeding disorder characterized by a low thrombocyte count, increased risk of bleeding, and fatigue. Immunoglobulin G (IgG) autoantibodies directed against platelet surface antigens can be detected in most patients with ITP. These autoantibodies accelerate platelet clearance, can inhibit platelet production, and may impair platelet function. Current ITP therapies include non-specific immunosuppression (steroids and rituximab), inhibition of platelet clearance (splenectomy, intravenous [IV] immunoglobulins, anti-D globulins, and syk inhibition) or stimulation of platelet production (thrombopoietin receptor agonists). However, steroids have known side effects, such as hypertension and fluid retention. Splenectomy provides sustained remission for a high proportion of patients, but has surgical and infection risks. IgG homeostasis is regulated by the neonatal Fc receptor (FcRn). Efgartigimod, a human IgG1 antibody Fc-fragment, is a natural ligand of the FcRn engineered to competitively bind to FcRn and prevent the recycling of endogenous IgG. In a Phase 2 trial in 38 patients with primary ITP, efgartigimod 5 mg/kg or 10 mg/kg given in 4 weekly IV infusions was well tolerated compared to placebo (Newland AC. Am J Hematol. 2020;95:178-187. NCT03102593). Efgartigimod induced a rapid reduction of total IgG levels associated with clinically relevant increases in platelet counts (up to 63.7% mean change from baseline 3 days after the fourth infusion), and a decreased proportion of patients with bleeding, compared with placebo. Platelet counts ≥50×109/L for >10 cumulative days were observed in 38% of efgartigimod patients and 0 placebo patients. Platelet counts ≥100×109/L were observed in 46% and 39% of patients receiving efgartigimod 5 mg/kg or 10 mg/kg and 1 (8%) patient receiving placebo. These results suggest that targeted IgG reduction with efgartigimod is a potential new treatment modality in primary ITP warranting longer-term Phase 3 evaluation.
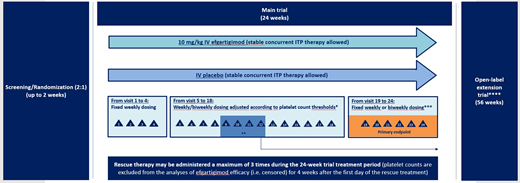
Study Design and Methods: ADVANCE, a Phase 3, multicenter, randomized, double-blinded, placebo-controlled trial (NCT04188379), will evaluate the efficacy and safety of efgartigimod in adults with persistent or chronic primary ITP. Eligible patients must have a mean platelet count <30×109/L over 3 qualifying counts and have received at least 2 prior ITP treatments or 1 prior and 1 concurrent treatment. Patients will enter a 24-week randomized period (2:1 ratio) and receive IV treatment with either efgartigimod 10 mg/kg or matching placebo. Permitted concurrent ITP treatments include oral corticosteroids, oral immunosuppressants, dapsone/danazol and/or eltrombopag. Randomization will be stratified by concurrent ITP treatments at baseline and history of splenectomy. Efgartigimod or placebo will be given weekly from visits 1 to 4 and then either weekly or every other week from visits 5 to 16, as determined by platelet counts. Dosing will be fixed at visit 16. The primary endpoint is the proportion of patients with a sustained platelet count response defined as platelet counts of ≥50×109/L for at least 4 of the 6 counts taken between weeks 19 and 24 (visits 20 and 25). Patients who discontinue treatment prior to visit 24 due to either lack of efficacy or an AE without achieving sustained response between visits 19 and 24, will be considered non-responders. Patients who receive rescue therapy at visit 12 or later, or for whom dose and/or frequency of concurrent ITP therapies have increased at visit 12 or later, will also be considered non-responders. Secondary endpoints include overall platelet count response, safety and tolerability, bleeding severity, use of rescue therapy, quality of life and patient-reported outcomes measures, and the immunogenicity and pharmacokinetic/pharmacodynamic effects of efgartigimod. Efficacy analyses will be performed on the full analysis set, consisting of all randomized patients who have a baseline efficacy observation. Safety will be assessed in all patients who receive ≥1 dose of study treatment.
Recruitment: ADVANCE recruitment is ongoing with a target of ~117 patients with chronic primary ITP and up to 39 patients with persistent primary ITP across approximately 80 sites in Europe, Japan, and the US. Trial participants will be eligible for continuation into ADVANCE+, a long-term open-label extension trial (NCT04225156).
Newland:Sobi: Other: Ad Board; Novartis: Other: Ad Board, Research Funding, Speakers Bureau; Dova Pharmaeceuticals: Other: Ad Board; Grifols: Other: Ad Board; argenx: Other: Ad Board; Amgen: Other: Ad Board, Research Funding, Speakers Bureau; GSK: Research Funding. Liebman:Dova: Consultancy; Pfizer: Consultancy; Rigel: Consultancy; Novartis: Consultancy; argenx: Consultancy; Bristol-Myers: Research Funding; Janssen: Research Funding. McDonald:Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Michel:Rigel: Consultancy; Novartis: Consultancy; Amgen: Consultancy. Miyakawa:argenx: Consultancy; Kyowa Kirin: Consultancy; Zenyaku Kogyo: Consultancy; UCB: Consultancy. Parys:argenx: Current Employment. De Haard:argenx: Current Employment. Ulrichts:argenx: Current Employment. Godar:argenx: Current Employment. De Beuf:argenx: Current Employment. Ayguasanosa:argenx: Current Employment. Broome:Alexion: Honoraria; argenx: Honoraria; apellis: Honoraria; sanofi: Honoraria. Kuter:Rigel: Consultancy, Honoraria, Other, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Actelion (Syntimmune): Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Protalex: Consultancy, Honoraria, Other, Research Funding; CRICO: Consultancy, Honoraria; Immunovant: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Principia: Consultancy, Research Funding; Protalix Biotherapeutics: Consultancy; Shionogi: Consultancy; Protalex: Consultancy, Honoraria, Research Funding; Shionogi: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Dova: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Kezar Life Sciences, Inc: Other, Research Funding; Merck Sharp Dohme: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; Alnylam: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Agios: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Amgen: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Zafgen: Consultancy, Honoraria; Up-To-Date: Consultancy, Honoraria, Patents & Royalties; UCB: Consultancy, Honoraria; Platelet Disorder Support Association: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Argenx: Consultancy, Honoraria, Other: Travel Expenses, Research Funding; Caremark: Consultancy, Honoraria; Immunovant: Other: Travel Expenses, Research Funding; Principia Biopharma: Consultancy, Honoraria, Other, Research Funding; Takeda (Bioverativ): Consultancy, Honoraria, Other, Research Funding; Sanofi (Genzyme): Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal